EPIGENETICS
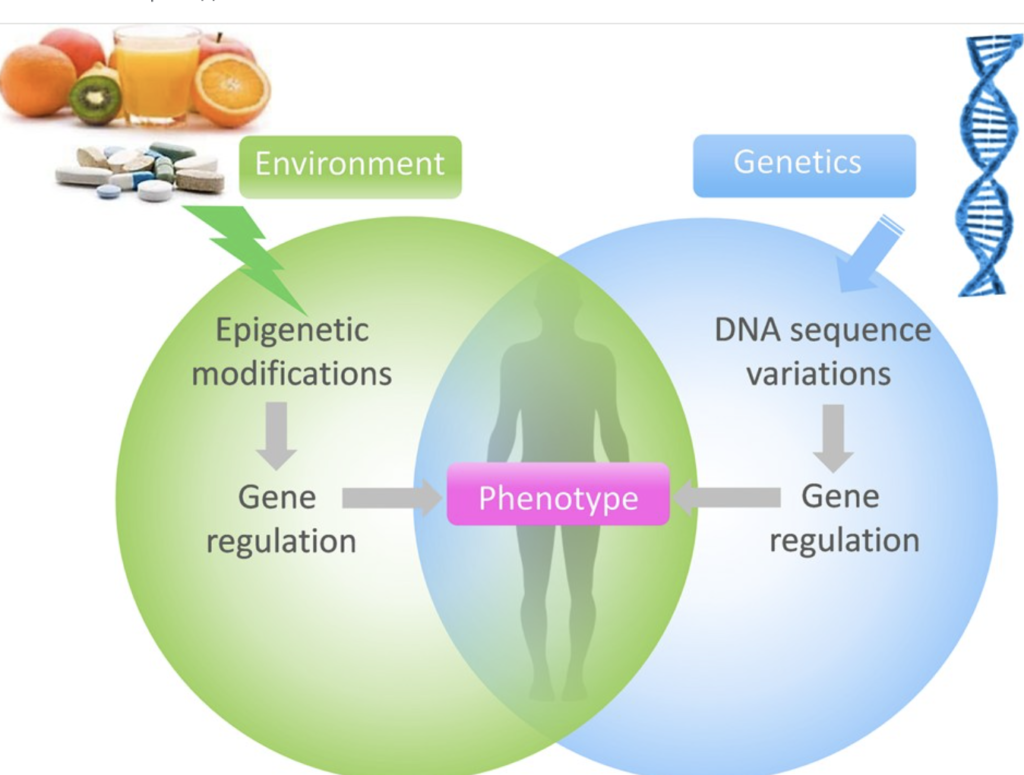
Your behavior and environment can lead to changes that affect how your genes work. Unlike genetic changes, epigenetic changes are reversible and do not change the DNA sequence, but they can change how your body reads the DNA sequence. In biology, epigenetics is the study of inherited changes in the phenotype that are not related to changes in the DNA sequence.
Tissue stem cells sense their environment, and this perception affects their future fate and function. Stem cells accumulate epigenetic memories of various environmental events. Stem cells carry the memory of their original niche, migration, encounters with inflammation, and adaptation to new fates and challenges. www.science.org/doi/10.1126/science.abh2444
Analysis of clones of individual stem cells showed that methylation changes during senescence are stochastic. The lifespan of MSC (mesenchymal stem cell) populations can vary greatly due to the accumulation of (random) epigenetic aberrations. onlinelibrary.wiley.com/doi/full/10.1111/acel.12544
Stem cell dysfunction is a sign of aging. Epigenetic changes play a critical role in the loss of stem cell function with age.
Recovery of nuclear acetyl-CoA via ectopic expression of CiC or addition of acetate to MSCs in culture rejuvenates MSCs, restoring the potential for efficient differentiation into an osteogenic lineage. Citrate, which has recently been reported to prolong lifespan in Drosophila, chemically includes acetyl-CoA and may be useful in restoring cytoplasmic and nuclear levels of acetyl-CoA. The general applicability of the CiC defect to old cells, especially stem cells, should be established.
Chromatin remodeling due to citrate degradation impairs osteogenesis of aged mesenchymal stem cells.
doi.org/10.1038/s43587-021-00105-8
Aging is accompanied by a general decline in the function of many cellular pathways. Older mesenchymal stem cells show reduced chromatin availability and lower histone acetylation, especially at osteogenic gene promoters and enhancers. The decrease in histone acetyl-I is due to impaired mitochondrial acetyl-CoA export due to lower citrate carrier (CiC) levels. In old cells, increased lysosomal degradation of CiC is observed, which is mediated by mitochondrial vesicles. Restoration of cytosolic levels of acetyl-CoA, either through exogenous expression of CiC or by addition of acetate, reconstructs the chromatin landscape and eliminates osteogenesis defects in old MSCs. The results show an age-dependent relationship between mitochondrial quality control, chromatin, and SC fate, which are linked through CiC. www.nature.com/articles/s43587-021-00105-8
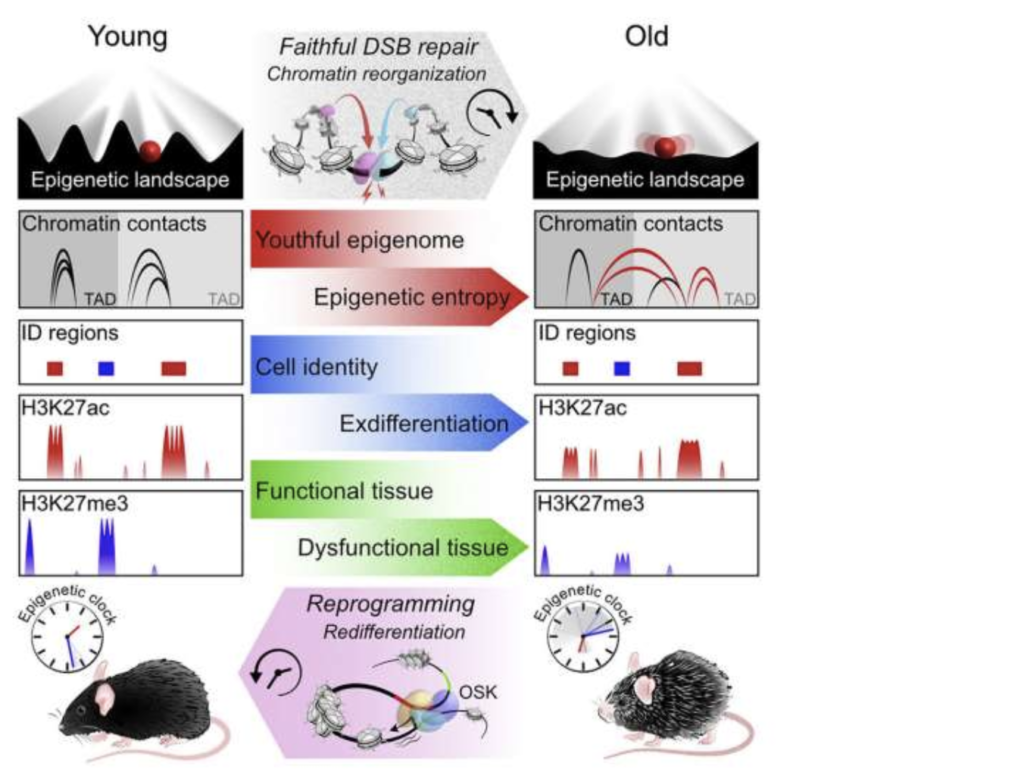
The 13-year study shows that the degradation of DNA organization and regulation, known as epigenetics, can cause aging in an organism independent of changes in the genetic code itself. The disruption of epigenetic information leads to aging in mice and that restoring the integrity of the epigenome reverses these signs of aging. This study is the first to show epigenetic changes as a major driver of aging in mammals.
www.cell.com/cell/fulltext/S0092-8674(22)01570-7
We are developing fundamentally new ways of epigenetic reprogramming by physical methods.
