Gene therapy ex vivo
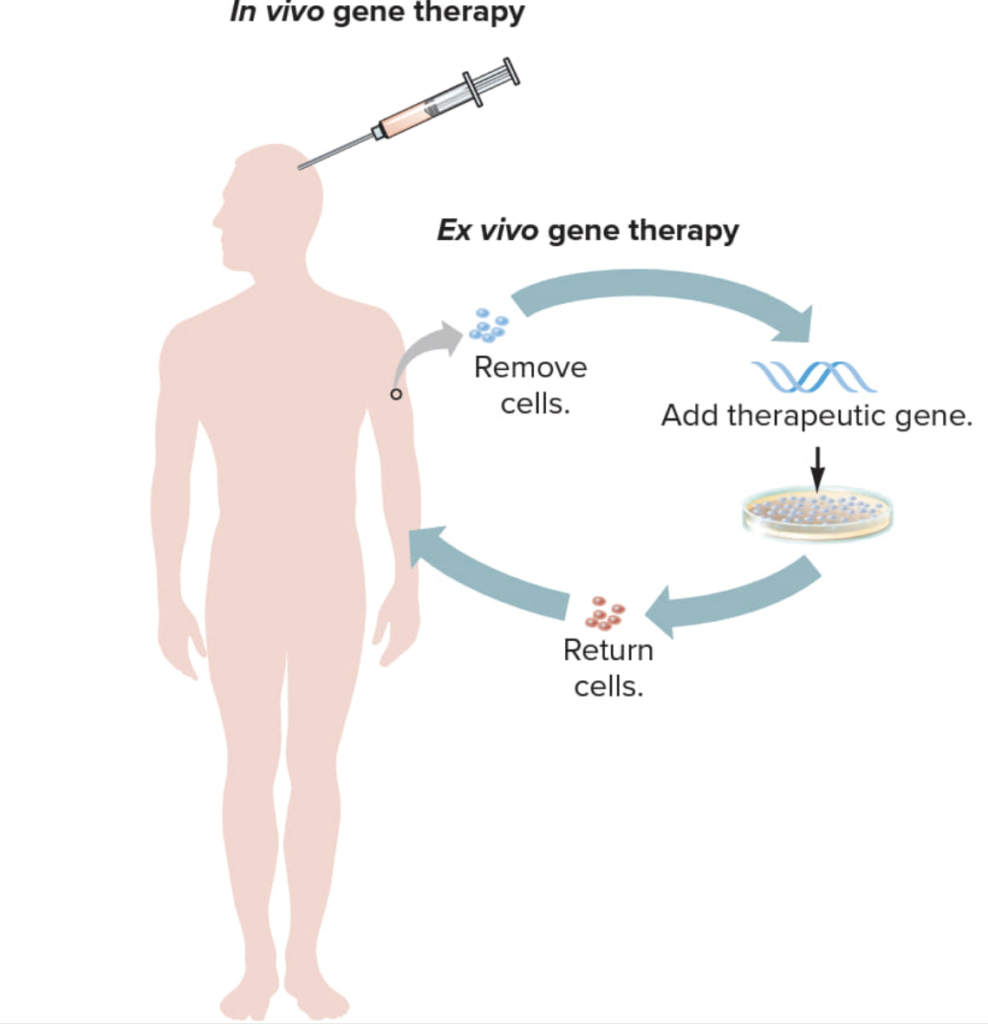
In ex vivo («out of body») gene therapy, cells are changed outside the body and then placed into the body, for example, through intravenous infusion or cerebrospinal fluid. But we have created more advanced technology.
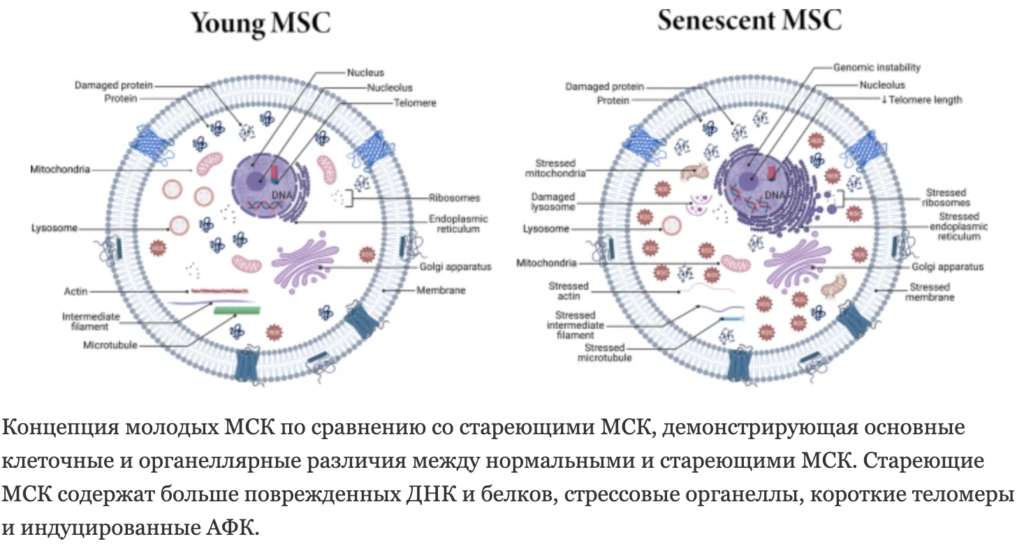
MSCs are of interest in clinical practice due to their immunomodulatory activity and ability to regenerate tissues. The first “switch” of the MSC aging mechanism is genomic errors. Genome instability, telomere shortening and epigenetic changes. Aging MSCs are characterized by a loss of DNA repair and antioxidant capacity, as a result of which they are more susceptible to oncogenesis and DNA damage.
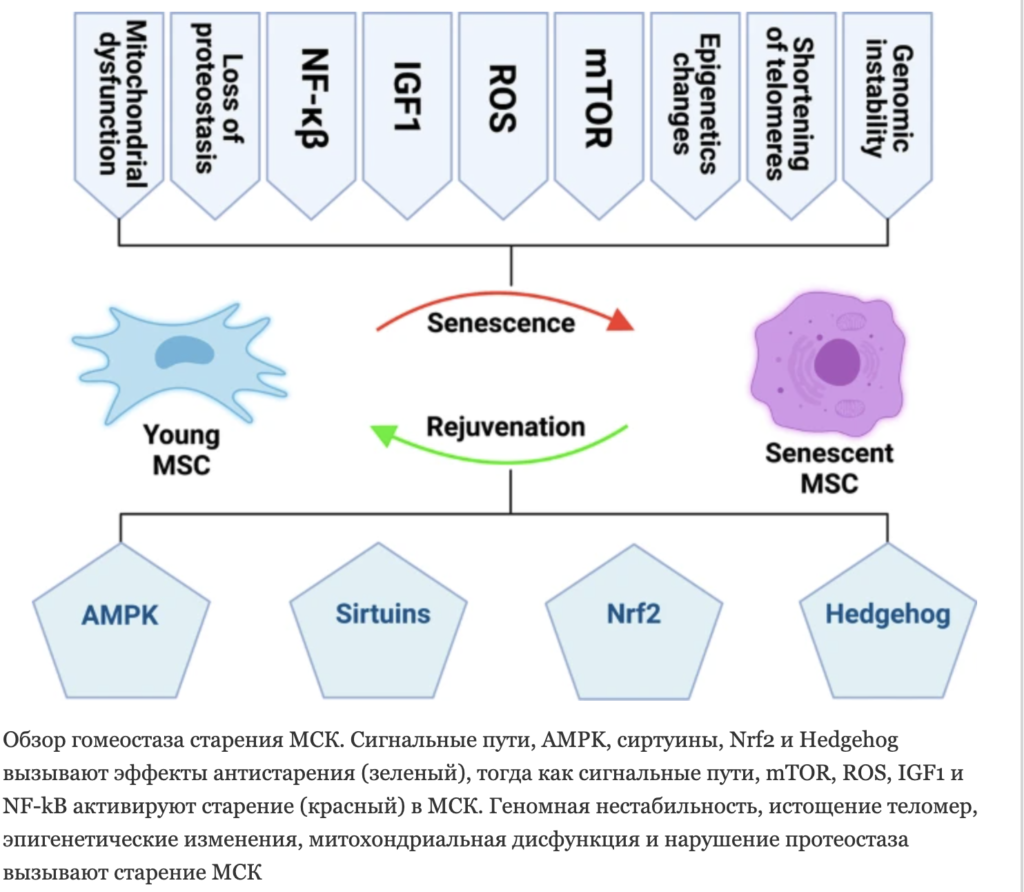
Aging MSCs are characterized by a loss of DNA repair and antioxidant capacity, as a result of which they are more susceptible to oncogenesis and DNA damage.
www.cmbl.biomedcentral.com/articles/10.1186/s11658-022-00366-0
A new technology for the restoration of the MSC genome (ex vivo) makes it possible to restore the lost age-related potential by restoring cell polyclonality.
Human aging alters the spatial organization between CD34+ hematopoietic cells and adipocytes in the bone marrow.
Age-related clonal hematopoiesis is a major risk factor for myeloid malignancy, and myeloid skew is a hallmark of aging.
Adiposity increases with age, which correlates with an increased density of mature myeloid cells and CD34+ hematopoietic stem/progenitor cells (HSPCs) and an increased proportion of HSPCs adjacent to adipocytes. However, the density and distance to HSPC and vessels remained stable. With aging, myeloid cell density increases in the hematopoietic areas surrounding adipocytes.
Increased adherence to adipocytes in the microenvironment may have affected the myeloid skew of aging HSPC, which contributes to age-related risk of myeloid malignancies. Aging increases bone marrow, myeloid, and CD34+ HSPC density in human bone marrow
• Human CD34+ HSPC niche is reticular, perivascular and periadipocytic in aging
• Aging increases the density of myeloid cells around adipocytes.
www.cell.com/stem-cell-reports/fulltext/S2213-6711(20)30232-0
In the body of a healthy person at birth, there are 50,000-200,000 HSCs, and they are constantly updated in this amount up to 25-30 years. At an early age, HSCs are found in the thymus, spleen, and bone marrow.
В организме здорового человека при рождении имеется 50 000-200 000 ГСК и они в этом количестве постоянно обновляются до 25-30 лет. В раннем возрасте ГСК находятся в вилочковой железе, селезенке, костном мозге.
After 25-30 years in the body of an adult, a transition occurs exclusively to bone marrow hematopoiesis. Since then, no new cells have been formed. And the supply starts to run out. Each cell in the billions of blood cells of one person forms its own clone of descendant cells, which form all immunity cells and circulating blood cells. Simultaneous work of up to 1000 HSC clones in the bone marrow forms a variety of blood cells that ensures human health and longevity.
The simultaneous systemic work of many HSC clones in the blood of one person is called polyclonalism. Each HSC clone has its own specifics during genomic analysis, although they all have the same genome, they have different exomes and epigenetic features by which they can be easily distinguished from each other. With age, the process of polyclonality changes. In addition, it is changed in healthy and sick people.
Hematopoietic stem cells or multipotent progenitor cells (HSC/MPPs) accumulate an average of 17 mutations per year after birth and lose 30 base pairs per year of telomere length. Hematopoiesis in healthy adults <65 years of age is massively polyclonal, with high clonal diversity and a stable population of 20,000–200,000 HSC/MPP uniformly contributing to blood production. On the contrary, hematopoiesis in individuals older than 75 years showed a sharp decrease in clonal diversity. In each of the elderly subjects, 30–60% of hematopoiesis was accounted for by 12–18 independent clones, each of which contributed to 1–34% of blood production.
Most of the clones began their expansion before the subject was 40 years old, but only 22% had known driver mutations. Genome-wide selection analysis showed that between 1 in 34 and 1 in 12 non-synonymous mutations were driving factors that accumulated at a constant rate throughout life and affected more genes than those found in blood cancers.
www.ncbi.nlm.nih.gov/pmc/articles/PMC9177428/
Moreover, the change in the clonality of bone marrow cells is directly related to the etiology of age-related changes in atherosclerosis. doi.org/10.1101/2022.01.18.476756
The TCR repertoire of CD8+ T cells remains relatively oligoclonal, and the same dominant TCR clones can be observed before and after aHSCT without significant clinical consequences. This suggests that these CD8+ T cells are not autoimmune or unable to induce disease activity after transplantation. CD4+ T cells are characterized by a complete renewal of the TCR repertoire, and especially increased TCR diversity for regulatory T cells seems important for successful induction of remission after transplantation. www.frontiersin.org/articles/10.3389/fimmu.2018.00767/full
Correction directions:
- adoptive immunotherapy with HSC;
- ex vivo gene therapy;
- the senile hematopoietic system can be restored with young mobilized peripheral blood, HSC. MicroRNAs in secreted microvesicles are responsible for improving hematopoietic functions by increasing the expression of genes targeting MYC and E2F and decreasing p53 expression.
www.aging-us.com/article/203689/text
WHY IS STEM CELL TRANSPLANTATION NOT ENOUGH?
The study used transplantation, parabiosis, plasma transfer, exercise, calorie restriction, and aging mutant mice to understand the effect of age-related systemic factors on HSCs and their bone marrow (BM) niche. It was found that neither exposure to young blood, nor prolonged stay in young niches after separation of parabionts, nor direct heterochronous transplantation had a noticeable rejuvenating effect on old HSCs. Similarly, exercise and calorie restriction did not improve the function of the old HSCs nor the old BM niches.
In contrast, juvenile HSCs were not affected by systemic conditions that promote aging, and HSC function was not affected by aging-affecting mutations in established long-lived or progeroid genetic models. Therefore, the circulatory system, which carries factors with rejuvenating or aging properties to many other tissues, is itself immune to these factors. That is why we recommend keeping your own HSCs at a younger age, a source of blood cells that can be transplanted at an older age.
www.rupress.org/jem/article-pdf/218/7/e20210223/1416480/jem_20210223.pdf
NEW STRATEGY
Intercellular transfer of telomeres
Cell-to-cell transfer of telomeres saves T-cells from aging and promotes long-term immunological memory. www.nature.com/articles/s41556-022-00991-z
STEM CELL NICHE REMODELING
The persistence of stem cells throughout life is tightly regulated by regulatory networks maintained by both intrinsic and extrinsic mechanisms (eg, microenvironmental communication, paracrine regulation mediated by cytokines and chemokines). In turn, the unique properties of each tissue stem cell type are formed by highly specialized niches that coordinate signals for adhesion, spatial organization, movement, self-renewal, or differentiation. Following a similar paradigm, stem cells connect to their niches to transition between passive and active states under physiological and pathological conditions. Indeed, a growing body of evidence suggests that niches are critical for long-term dormancy maintenance, as they link internal mechanisms, microenvironment interactions, and communication with surrounding cells together to balance dormancy, stem cell proliferation, and regeneration.
Despite their resilience, stem cells accumulate damage over time, which ultimately reduces the number and function of stem cells. Recent studies show that quiescent stem cells are particularly vulnerable to aging-related damage.
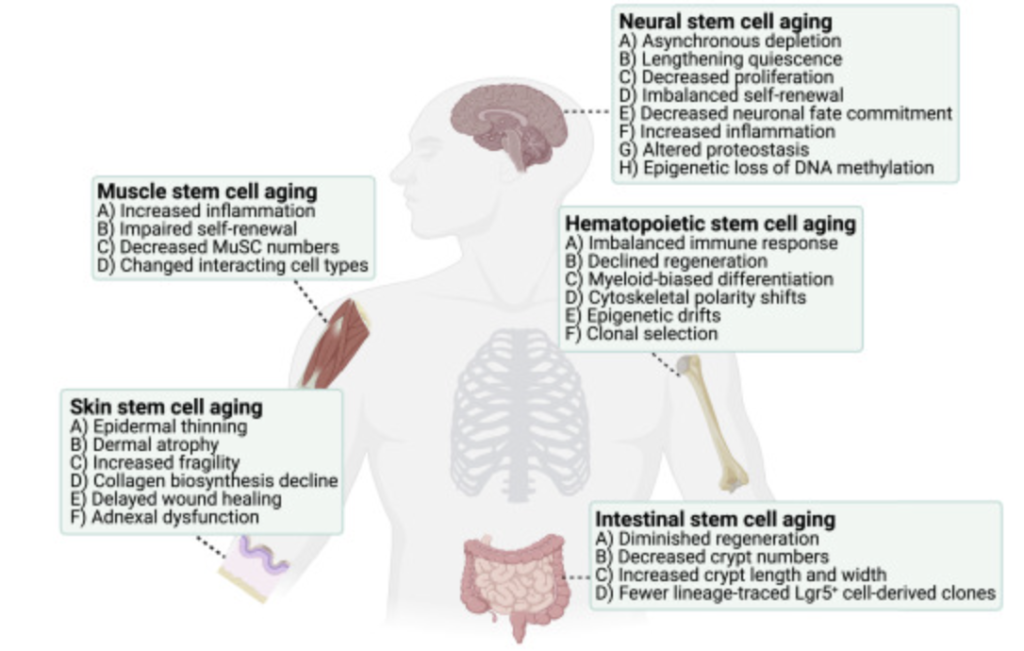
Clonal selection of HSCs also underlies the mechanisms of aging, coupled with marked changes in HSC niches.
www.cell.com/cell-reports/fulltext/S2211-1247(22)01292-X
We have developed a new method of EX VIVO therapy targeting (based on analyzes) restoring polyclonality. This is critical because aged HSCs were resistant to systemic blood-borne rejuvenation interventions such as transplantation, parabiosis, and plasma transfer.
www.cell.com/cell-reports/fulltext/S2211-1247(22)01292-X
NK Cells
NK cells (or Natural Killer Cells) are important immune cells that are critical for innate immunity. They have anti-tumor, anti-viral, and immune regulatory functions. NK cells are the patrol units of the immune system, and they can respond quickly to kill diseased cells. The most common method of NK cell therapy is to obtain human peripheral blood mononuclear cells and to subject these to antibody and cytokine stimulation ex vivo, resulting in vitro NK cell activation and proliferation.
NK Cells for Anti-aging by Senescent Cell Removal: As senescent cells are naturally targeted for elimination by NK cells, it could be beneficial to use NK cells to eliminate persistent pro-inflammatory senescent cells, particularly as they accumulate during aging. The broad cytotoxicity and rapid killing ability make NK cells ideal for use in immunotherapy and to support healthy aging.

